|
PinkMonkey Online Study Guide-Biology
2.1 Structural Organization and the Chemical Basis of Life
(A) Introduction
The universe and living bodies are composed of
matter which occupies space and possesses mass. Matter can
exist in four forms -solid, liquid, gaseous and plasma. Matter is
made up of basic substances called elements. There are over
a hundred elements recognized by scientists today. An element is
made up of identicle atoms. Calcium, Carbon, hydrogen, iron, sodium,
etc. are some elements.
i) Atoms
Each element is made up of one particular
kind of atom. Atoms are the smallest part of an element which do
not share the properties of the element. An atom consists of 3 basic
particles:
a) Protons, which are positively charged
and are present in the nucleus.
b) Electrons, which are negatively charged
and which rotate around the nucleus.
c) Neutrons, which are present in the nucleus
and which donít have any electricle charge. A neutron has approximately
the same mass as a proton.
In the nucleus of the atom the proton and neutron are
firmly attached to each other. The chemistry of the atom is dependent
upon the number of protons and electrons in the atom. Protons and electrons
are present in the same number in the atom, which leads to a neutral electrical
charge in the atom. The nucleus of an atom is surrounded by shells or
orbits of electrons. In the first orbit there are a maximum of 2 electrons.
From the second shell to the last shell, every shell has a maximum of
8 electrons. When the final orbit of an atom is complete with electrons
it becomes a stable atom. When there is a single electron or more missing
in the last orbit of the atom, the atom becomes active allowing for a
chemical reaction with another atom. During the reaction there is sharing
or exchange of electrons. Atoms are most stable when they have a full
outer shell. If they can, they will either share one or more electrons
with another atom so that both atoms " think " they have full
outer shells, or sometimes an atom that " needs " one or two
electrons to have a full outer shell will actually take electrons another
atom that has only one or two electrons in its outer shell; therefore,
both atoms end up with full outer shells.
ii) Molecules
In biology we study molecules as part of
molecular biology, molecular interaction, etc. Atoms combine chemically
in a specific order to form molecules. For instance, two atoms of
hydrogen combine with one atom of oxygen to form a single molecule
of water. A molecule is the smallest particle of a substance existing
freely yet retaining the characteristics of that substance. A collection
of molecules forms a compound. Properties of the compound depend
upon molecules and atoms present in the molecule. Consider this
example: the molecular weight of the compound, water, is 18. This
weight has been calculated taking into account the weight of its
molecular components H and O.
H2O ®H + H + O ® 1 +1+ 16 ® 18
Some molecules are formed from atoms of the same element
e.g. oxygen molecule (O2) is formed from 2 atoms of oxygen;
ozone (O3) is formed from 3 atoms of oxygen. But, oxygen or
ozone cannot be called a compound because in a compound we require atoms
of different elements.
When the compound is formed it contains different elements.
These elements stay together by means of links between them. In scientific
terminology this link is called a bond. There are various kinds
of bonds. In a compound, when one atom of an element gives away an electron,
this exchange creates a bond between these two elements. This is an ionic
bond. This bond consists of an electromagnetic force which is formed
due to exchange of charge [electron]; the atoms conducting the exchange
are called ions. This eletromagnetic force attracts two opposite
charges: a positive charge in the atom which gives away electrons and
a negative charge in the atom which takes up electrons. This attraction
force forms the ionic bond.
A second important type of bond is called the covalent
bond. Such a bond is formed when two atoms share one or more
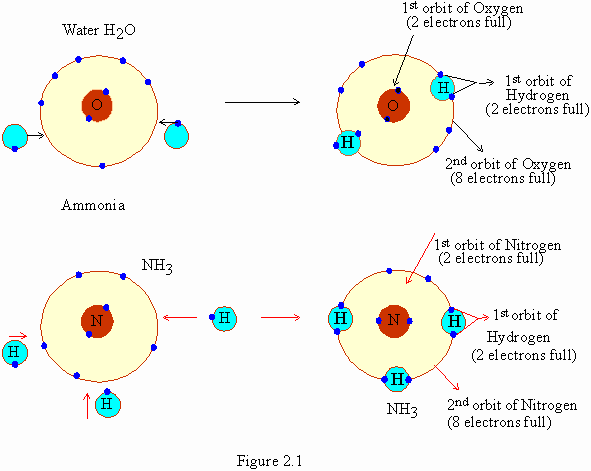
electrons with one another. For example (fig. 21), in water (H2O)
2 atoms of hydrogen share 2 electrons with oxygen. In ammonia 3
hydrogen atoms share one electron each with a single nitrogen atom.
This leads to the formation of ammonia (NH3). Between
atoms when one pair of electrons is shared, a single covalent
bond is formed. When two pairs of electrons are shared a double
covalent bond is formed.
Click here to enlarge
iii) Acids and Bases or Alkalis
If a compound reacts with water and releases
hydrogen ions (H+) ions then this compound is called an acid,
and is said to be acidic in nature. For example, when hydrogen
sulphide is mixed with water it releases hydrogen ions and the solution
becomes one of sulphuric acid. Other chemical compounds when dissolved
in water attract hydrogen atoms. These substances are called bases
or alkalis. For example, when sodium hydroxide (NaOH) is
mixed with water, sodium hydroxide attracts hydrogen ions from H2O,
and (OH-) ions remain. So these substances that remove (H+)
from water act as bases or alkalis.
|