|
Ions are atoms ( or groups of atoms ) which carry a positive
or a negative charge and become free and mobile when an electric
current is passed through an aqueous solution. Depending on the
type of electric charge carried by ions they are classified as

Click here to enlarge
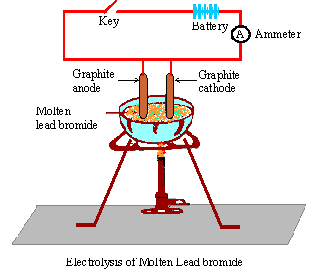
Example of Electrolysis
Electrolysis of Molten Lead Bromide

|
Index
9.1 Introduction
9.2 Electrolytic Cell
9.3 Electrolytes
9.4 Faraday's Law of Electrolysis
9.5 Electrochemical Cell
9.6 Electrode Potential
Chapter 10
|