|
9.6 Electrode Potential
Electrode Potential is denoted as EMF ( electro motive force
). EMF of any electrolytic cell is the sum of the EMF produced
at two electrodes.
EMF cell = Higher reduction potential - Lower reduction potential
Potential of a single electrode cannot be measured directly. It is measured by connecting reference electrode ( with known electrode potential ).
Table 13
Standard electrode potential on Hydrogen scale at 298K

Click here to enlarge
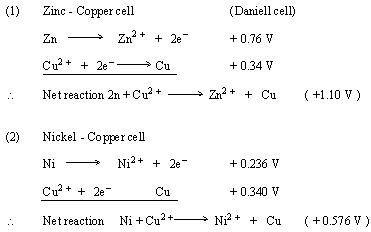
Similarly the standard potential of any electrode can be determined if the EMF of the cell is given. To calculate the standard potential of the zinc electrode if EMF of the cell is 1.559 V in Zinc - Silver electrode,

[next chapter]
|