|
12.6 Dissociation
Degree of dissociation
It is a fraction of the total number of moles of an acid or base or electrolyte that dissociates into ions in an aqueous solution when equilibrium is reached. It is represented by a

Thus, greater the degree of dissociation (a) stronger the acids or bases and vice versa.
Dissociation constants
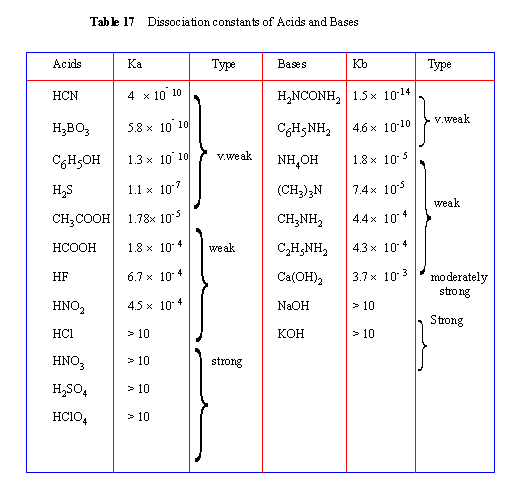
Strong acids and strong bases dissociate in a solution almost completely. Hence the dissociation studies are important only with respect to weak acids and weak bases.
Dissociation of weak acids : Consider a weak acid HA which dissociates in water as
HA + H2O
 H3O+
+ A- H3O+
+ A-
For simplicity it can be written as HA  H+ + A- H+ + A-
Applying the law of mass action

Where Ka is known as acid ionization constant (or acid constant). Higher the value of Ka greater the degree of dissociation.
\ Acid with high Ka
value are relatively stronger than acid with lower Ka
value.
Dissociation of weak bases : Consider a weak base BOH which dissociates as
BOH  B+ + OH- B+ + OH-
Applying the law of mass action
 Where Kb is known as base ionization constant (or base constant). Higher the value of Kb greater the degree of dissociation. Where Kb is known as base ionization constant (or base constant). Higher the value of Kb greater the degree of dissociation.
\A base with high Kb value is relatively stronger than a base with lower Kb value.
An acid or base that has Ka or Kb values greater than 10 are considered to be strong acids or bases. Values between 10- 2 to 10- 3 are regarded as moderately strong, while those between 10- 4 to 10- 7 as weak and those with values less than that are regarded very weak acids or bases.
Click Here To Enlarge
[next page]
|