|
PinkMonkey Online Study Guide-Biology
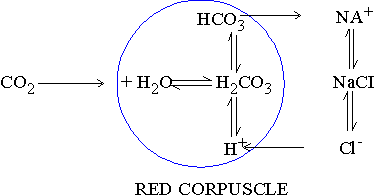
Figure 17.9 Chloride shift
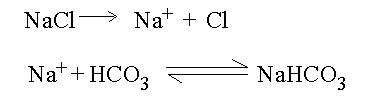
As bicarbonate ions (HCO3)
diffuse into plasma from RBC's, Hydrogen ions (H+)
concentration in RBCs increases. For maintaining ionic balance for
each bicarbonate (HCO3)
one chloride ion (Cl) from plasma enters the RBC. This is called
"chloride shift". H+ ions in RBC's also bring about
dissociation of oxyhemoglobin (HbO2)
into Hb and O2.
The oxygen released diffuses into the tissues while hemoglobin buffers
hydrogen to form very dilute hemoglobinic acid.
(ii) About 10% of CO2
combines with an amino group (NH2)
on hemoglobin to form carbaminohemoglobin. This enters the lungs and dissociates
into hemoglobin and CO2,
the latter of which then diffuses into the alveoli.
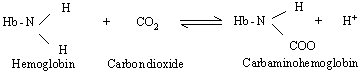
(iii) Nearly 5% of CO2 never enters RBC but dissolves in plasma, where it combines with plasma protein to form proteinic acid which is carried to the lings where it dissociates into protein and CO2. CO2 further enters alveoli of the lungs.
In the lungs CO2
tension is less than in the blood stream, hence the reversible reactions
take place. Bicarbonate enter RBC and because of the enzyme carbonic anhydrase,
it dissociates into H2O
and CO2.
This dissociation is facilitated by acidic oxyhemoglobin present at the
respiratory surface. As a result CO2
diffuses into alveoli and is expelled during respiration.
**********
|
Table of Contents
17.0 -
Introduction
17.1 -
Gaseous Exchange and Transport
Chapter
18
|