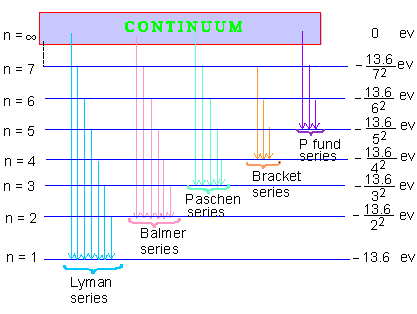
The success of Bohr's formula as per Equation (9), does not lie only in predicting value of R and accounting for Balmer's formula but in predicting the existence of various series of spectral lives in an hydrogen atom spectrum. The first five such series have been detected and are as follows :
1. Lymn Series : Ultra- violet range
2. Balmer Series : Visible range
3. Paschen Series : Infra-red range
4. Bracket Series : Higher Infra-red range
5. Pfund Series : For Infra-red range
Energy level diagram for the Hydrogen atom Spectrum
Figure 1
|
32 : Bohr's theory of hydrogen
atom and its spectrum Follow @Pinkmonkey_com  |